PART IV: Molarity. 2 Review: How to you go from grams of a substance to moles (and vice versa)? Use molar mass! Ex: How many moles are in 0.50g NaCl? - ppt download

Phlogopite static CA results with pressure and temperature change at... | Download Scientific Diagram

If relative decrease in vapour pressure is 0.4 for a solution containing 1 mol NaCl in 3 mol H2O . NaCl is

Calculate the mass of 1 mole of each one of the following: (a) `NaCl` , (b) `CaCO_(3)` , (c ) `FeSO - YouTube

SEM images of the samples electro-deoxidized in the CaCl 2-NaCl-1mol%... | Download Scientific Diagram
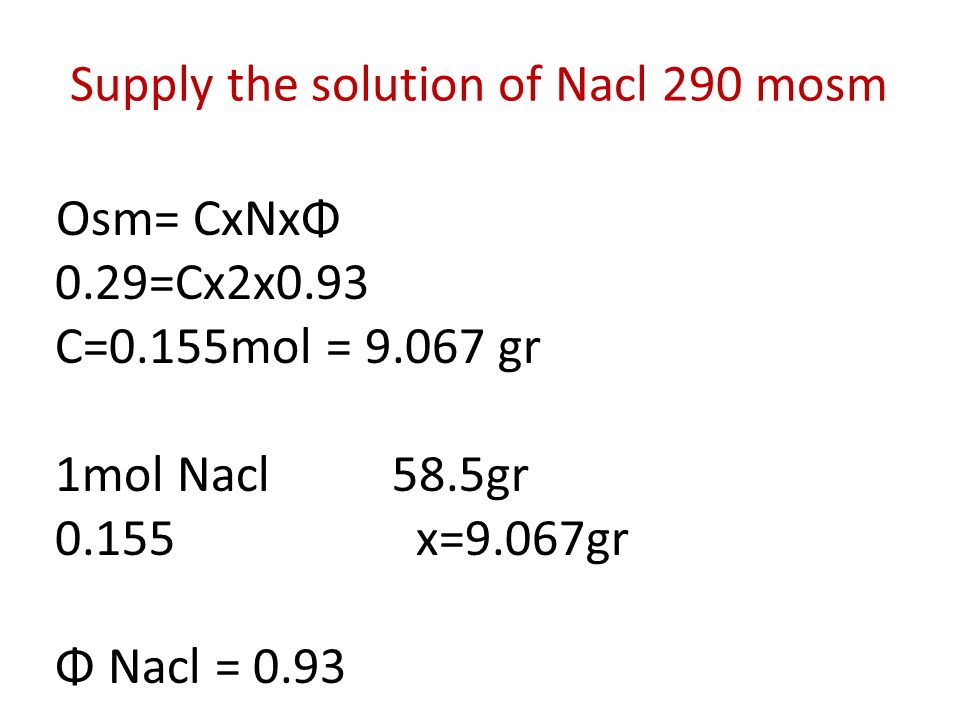
Osmosis 1mol=1moleculgram disolved particle Osmol : 1Osmol =1mol disolved particles 1mol=1moleculgram disolved particle. - ppt download

Effects of 1 mol/L NaCl and the 1 mol/L NaNO3 electrolytes on surface... | Download Scientific Diagram
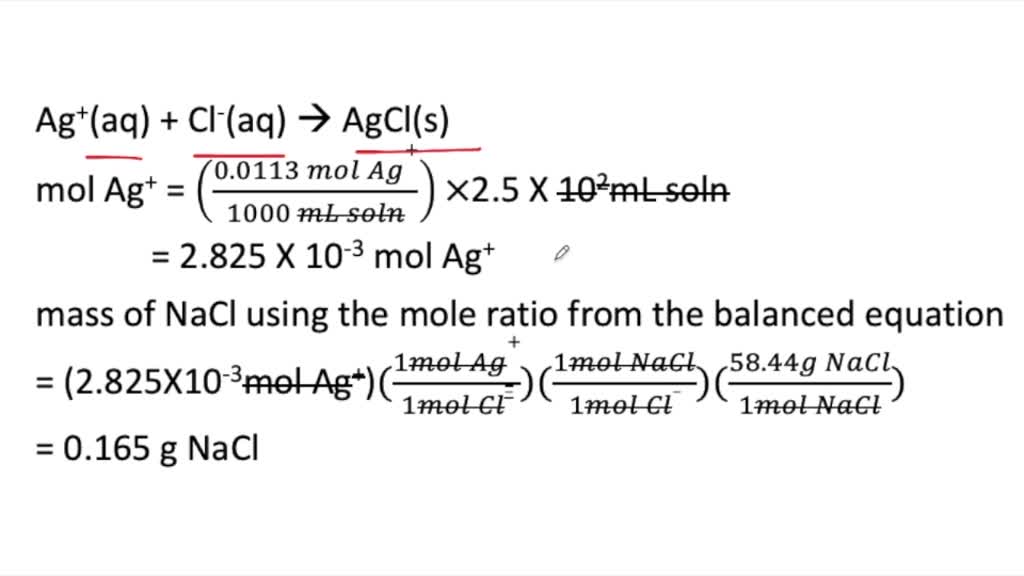
SOLVED:How many grams of NaCl are required to precipitate most of the Ag ions from 2.50 ×10^2 mL of a 0.0113 M AgNO3 solution? Write the net ionic equation for the reaction.

Calculate the Gibb's energy change when i mole of NaCl is dissolved in water at 25^∘ C. Lattice energy of NaCl = 777.9 KJ mol^- 1 , Δ S for dissolution =

Explain in why on addition of 1 moe of NaCl to 1L of water, the boiling point of water increases, - YouTube

Concentration Calculations Molarity. Objectives To calculate the molecular weight and moles of a substance To calculate the Molarity of a substance using. - ppt download