Free Online Help: Given the following delta H values H2+1/2O2--->H2O delta H =-285.8 H2O2---->H2+O2 delta H = 187.6 Calculate delta H rxn for the following reaction H2O2--->H2O + 1/2O2

Measured (fitted) diffusion resistances in H2O/H2 and CO2/CO mixtures... | Download Scientific Diagram
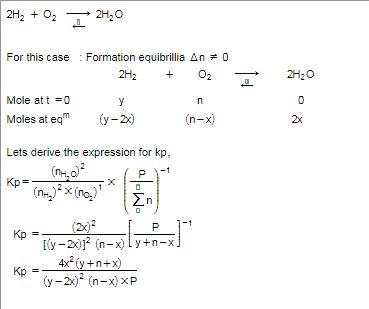
n mole each of h2o h2 o2 r tken in closed container at temperature t if y mole of h2 r disasssociated at equillibrium n equillibrium pressure is p the cgt66gee -Chemistry -

Consider the following reaction at certain temperature: H2O(g)+CO2(g) equilibrium to H2(g)+CO2(g) Some molecules of H2O and CO are placed in a 1.0 L container as shown below. When equilibrium is reached, how

calculate the equilibrium constant of H2 + O2 gives us H2O + CEO at 13957 if the equilibrium constant 135 - Chemistry - Equilibrium - 13886927 | Meritnation.com
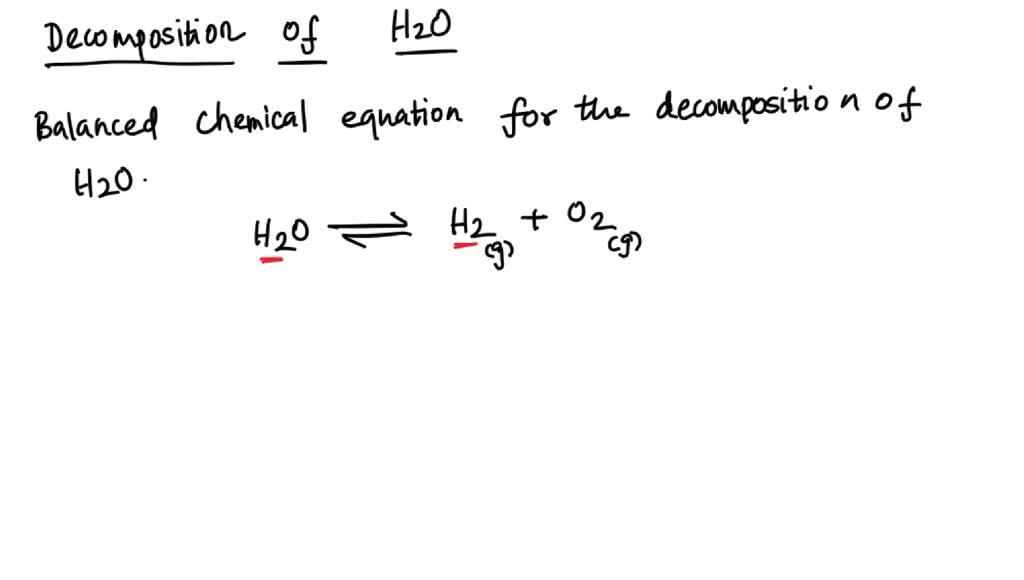
SOLVED: The decomposition of water into hydrogen gas H2 and oxygen gas O2 can be modeled by the balanced chemical equation A) H2 + O2 → H2O B) H2O → H2 +

![PDF] Initiation in H2/O2: Rate constants for H2+O2→H+HO2 at high temperature | Semantic Scholar PDF] Initiation in H2/O2: Rate constants for H2+O2→H+HO2 at high temperature | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/59651c1a79d5be73d45e3b492f6f4396965dd05f/5-Table1-1.png)