
Kal(so4)2.12h2o Aluminium Potassium Sulfate Potassium Alum Powder - Buy Potassium Alum Powder,Aluminium Potassium Sulfate Powder,Kal(so4)2 12h2o Potassium Alum Product on Alibaba.com

How to Balance Al2O3 + H2SO4 = Al2(SO4)3 + H2O,How to Balance Aluminum oxide + Sulfuric acid,How to Balance Aluminum oxide + Sulfuric acid + Aluminum sulfate + Water,balancing Al2O3 + H2SO4 =
![Y 3+ is coordinated by nine oxygen atoms in NaY[SO4]2 • H2O with C2... | Download Scientific Diagram Y 3+ is coordinated by nine oxygen atoms in NaY[SO4]2 • H2O with C2... | Download Scientific Diagram](https://www.researchgate.net/publication/351749785/figure/fig2/AS:1027001976635392@1621867760325/Y-3-is-coordinated-by-nine-oxygen-atoms-in-NaYSO42-H2O-with-C2-symmetry-left-and.png)
Y 3+ is coordinated by nine oxygen atoms in NaY[SO4]2 • H2O with C2... | Download Scientific Diagram
![SOLVED: [Cu(NH3)4]SO4 • H20 (s) was prepared from 15.5 grams CuSO4 • 5 H2O (s) using a total of 10 mL of 15 M NH3. Determine which reactant is limiting. Molar Masses: SOLVED: [Cu(NH3)4]SO4 • H20 (s) was prepared from 15.5 grams CuSO4 • 5 H2O (s) using a total of 10 mL of 15 M NH3. Determine which reactant is limiting. Molar Masses:](https://cdn.numerade.com/ask_previews/3e5daa89-75dc-40aa-92e7-425d1e093c86_large.jpg)
SOLVED: [Cu(NH3)4]SO4 • H20 (s) was prepared from 15.5 grams CuSO4 • 5 H2O (s) using a total of 10 mL of 15 M NH3. Determine which reactant is limiting. Molar Masses:

How to Balance H2SO4 + B(OH)3 = B2(SO4)3 + H2O | How to Balance H2SO4 + B(OH)3 = B2(SO4)3 + H2O | By Organic Chemistry Tutorial/Inorganic Chemistry/Science | Facebook

Balance the following chemical reactions (i) Fe + H2SO4 → Fe2(SO4)3 + H2 (ii) C2H6 + O2 → H2O - Science - Chemical Reactions and Equations - 16678311 | Meritnation.com
, RE(SO4)[B(OH)4](H2O)2, and RE(SO4)[B(OH)4](H2O)·H2O: Rare-Earth Borate-Sulfates Featuring Three Types of Layered Structures | Inorganic Chemistry RE(SO4)[B(OH)4](H2O), RE(SO4)[B(OH)4](H2O)2, and RE(SO4)[B(OH)4](H2O)·H2O: Rare-Earth Borate-Sulfates Featuring Three Types of Layered Structures | Inorganic Chemistry](https://pubs.acs.org/cms/10.1021/acs.inorgchem.7b02317/asset/images/large/ic-2017-02317c_0008.jpeg)
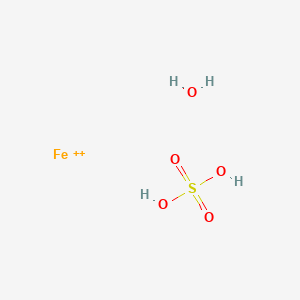
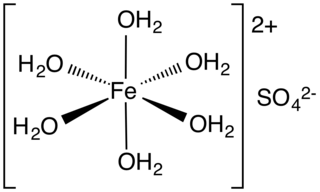





![Thermal effects in [CsEu(H2O)3(SO4)2]·H2O. | Download Scientific Diagram Thermal effects in [CsEu(H2O)3(SO4)2]·H2O. | Download Scientific Diagram](https://www.researchgate.net/profile/Aleksandr-Oreshonkov/publication/354150483/figure/tbl1/AS:1061074447114240@1629991270579/Thermal-effects-in-CsEuH2O3SO42H2O_Q320.jpg)


